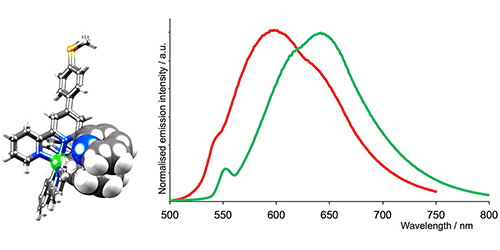
1) Light harvesting using inorganic coordination complexes as dyes in dye-sensitized solar cells (DSCs)

We focused on the development of dyes (sensitizers) for attachment to n-type semiconductors (usually TiO2) for incorporation into DSCs. The now well-established Grätzel-type DSCs use ruthenium(II)-containing dyes, and our research thrust was the development of dyes that contain first row d-block metals (e.g. copper, iron, zinc) which are Earth abundant. The major challenge is to increase the sunlight-to-electrical-power conversion efficiencies of the dyes.
For an overview of this area:
The emergence of copper(I)-based dye sensitized solar cells
C. E. Housecroft and E. C. Constable
Chem. Soc. Rev., 2015, 44, 8386-8398. doi.org/10.1039/C5CS00215J
The versatile SALSAC approach to heteroleptic copper(I) dye assembly in dye-sensitized solar cells
F. J. Malzner, C.E. Housecroft and E.C. Constable
Inorganics, 2018, 6, 57. doi.org/10.3390/inorganics6020057
See also our most recent publications under 'Publications'.